
Biostatistics & Data Management
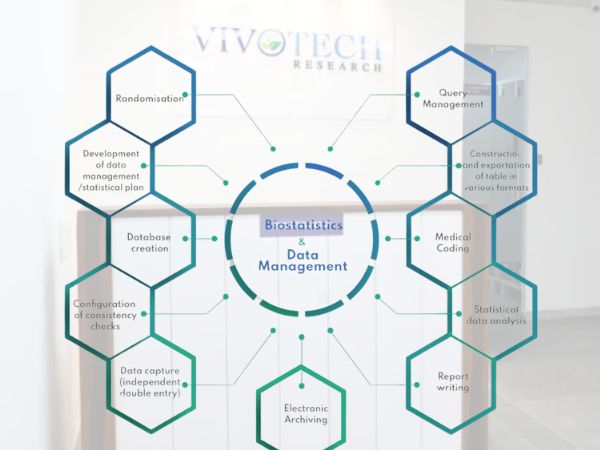
- An experienced team to help manage studies across a variety of industry standard EDC platforms
- Knowledge on EDC technology and surrounding processes and how to ensure the integrity of every data set
- Case report form (CRF) design, electronic or paper
- Database set-up and validation
- Electronic data capture (EDC) systems implementation and training
- Double data entry for paper CRF–based studies
- Data sets and TLFs based on CDISC compliant specifications
- In-depth, thorough quality assessments, checking for errors or trends and querying data through final database lock
- Sample size calculations, randomization schedules, statistical analysis plans (SAP), data interpretation, and more
